Latent Heat and specific heat: examples and solved problems
Latent Heat

Ice crystal - see larger image
(We consider isolated systems, unless otherwise stated, so that no heat is lost to the environment and the principle of conservation of energy can be applied to work out the answer.)
1) How much heat is needed to transform 500g of ice at -20 oC into water at 50 oC ? (cice = cwater / 2) . Table of latent heats here.
2) a) 4 kg of water (at 25 oC) and 2 kg of ice (at 0 oC) are mixed inside an isolated container. What is the final temperature ? Is there any ice left?
b) What happens if there was only 1 kg of ice?
3) Two ice cubes at 0ºC are inserted in a recipient containing water at 25ºC. As a result, the temperature of the water goes down to 1ºC and the ice cubes completely melt. What would happen if 4 ice cubes were inserted, instead of 2? You would find:
a) only water above 0ºC
b) only water at 0ºC
c) ice at 0ºC and water above 0ºC
d) ice and water at 0ºC
e) only ice at 0ºC
4) This exercise is and exception in the sense we don´t have an isolated container. This container contains ice in thermal equilibrium with water .Heat is being lost to the environment acoording to the graph below. Based on the graph, the rate of heat transfer to the outside of the container is aproximately:
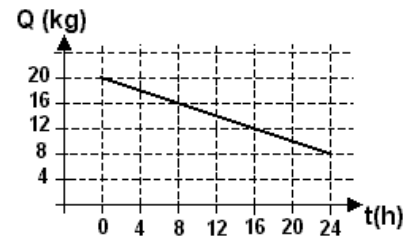
Latente heat of fusion of ice ≈ 320 kJ/kg
a) 0,5 kJ/h b) 5 kJ/h c) 120 kJ/h d) 160 kJ/h e) 320 kJ/h
Answers:
1)This is done in 3 stages, each one of them taking a different amount of heat (Q):
Q1 = heating up the ice from -20 oC to 0 oC
Q2 = melting the ice
Q3 = heating up the water (molten ice) from 0 oC to 50 oC
------------------
Q1 = c m ΔT = 2093*0.5*20 = 20930 J
Q2 = Lf m = 3.33 x 105 *0.5 = 170000 J
Q3 = c m ΔT = 4186*0.5*50= 104650 J
The total heat taken is Q1+Q2+Q3 = 295580 J = 295.58 kJ
Interesting to observe that melting the ice is the step that takes the most energy (heat).
2) a) Firstly, let's check how much heat is needed to melt 2 kg of ice. The ice is at 0 oC (unlike the previous question) so that in this case it is only necessary to compute the latent heat . According to the table :
Lf = 3.33 x 105 J/kg. There are 2kg so that the total heat needed is :
Q1 =Lf m =3.33 x 105 *2 = 6.66 x 105 J.
-------------------
Next, let's check how much heat is needed to bring all the water to 0 oC :
cwater = 4186 J/kg oC ,
Δt = 25 , m = 4 kg, so:
Q2= c m ΔT= 4186*4*25 = 418600 J = 4.186 x 105
-----------------
Conclusion: There will be ice left, because Q2 is lower than Q1. The mixture will stay at 0 oC and no further heat transfers will occur (heat can only be transferred if there is a temperature difference).
As you can see, many problems mix the concepts if specific heat and latent heat.
2) b)
Q1 =Lf m =3.33 x 105 *1 = 3.33 x 105 J
In this case , Q1 < Q2 so that all the ice will be melted.
What will be the final temperature of the mixture? We know that it will be somewhere between 0 oC and 25 oC, so that we can use a procedure similar to question 3 in the previous page : The molten ice is treated as 1 kg of water at 0 oC which is mixed with 4kg of water at 25 oC.
4186*4*(25-Tf) = Q1 + 4186*1*(Tf-0)
418600 - 16744 Tf = 333000 + 4186 Tf
-20930 Tf = - 85600
Tf = 4.1 oC
3) This question doesn´t require any calculations. Only a knowledge of the concepts of specific heat and latent heat is necessary.
Initially 2 ice cubes are inserted and heat flows from the water to the ice. As a result, the all the ice melts and the temperature of the water goes down by 24 degrees. If 2 more cubes are inserted (making a total of 4), heat will again flow from the water (at 1 oC) to the ice until all the water is at 0 oC, and a little bit of ice will melt in this process.Not much because to melt 2 cubes completely the water temperature must go down by 24 degrees.
In conlcusion, ice will be left intact and we will end up with a mixture of ice and water, both at 0 oC.
Answer: d)
4) This question only require a knowledge of graph interpretation and the concept of latent heat .
According to the graph, 12 kg of ice melted in 24 h.
The amount of heat needed to do that (Q) is given by this mass times the latent heat of fusion of ice:
Q = 12 kg * 320 kJ/kg = 3840 kJ
To calculate the rate per hour we divide that by 24:
3840/24 = 160 kJ/h
Answer: d)
more exercises of specific heat>>
example - 9/11 physics- how much heat needed to weaken the towers?


